Acetaminophen Recall – 11 Million Bottles Possibly Tainted With Metal Bits
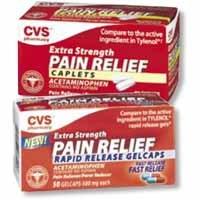 The maker of most store brand acetaminophen has announce a recall of over 11 million bottles of the drug that is mainly sold as a store brand. The pills were tainted with bits of metal from a 3rd party supplier the company stated. While there has been no one hurt by the medicine yet, this is something to be on the lookout for.
The maker of most store brand acetaminophen has announce a recall of over 11 million bottles of the drug that is mainly sold as a store brand. The pills were tainted with bits of metal from a 3rd party supplier the company stated. While there has been no one hurt by the medicine yet, this is something to be on the lookout for.
Perrigo Co. said it discovered the metal bits during quality-control checks.
The recall affects bottles containing various amounts of 500-milligram caplets.
Perrigo bills itself as the world’s largest manufacturer of store-brand non-prescription drugs. The Allegan, Mich., company did not disclose the chains for which it manufactures the store-brand acetaminophen.
However, Wal-Mart Stores Inc., CVS Corp., Walgreen Co. and Costco Wholesale Corp. are among the companies it supplies with health care products, according to company Securities and Exchange Commission filings.
Perrigo said the pills contained raw material purchased from a third-party supplier and affected 383 batches.
Acetaminophen is best known as the drug in products sold under the Tylenol brand, but is widely available in generic versions. The recall does not affect Tylenol. The recall should not cause a shortage of acetaminophen, the Food and Drug Administration said. via USATODAY.com.
If you liked this post, you may also like these:
Comments
2 Responses to “Acetaminophen Recall – 11 Million Bottles Possibly Tainted With Metal Bits”
Leave a Reply

 RSS
RSS





Why we need to be careful,with generics.
If they found the metal during a quality control check, does that mean they do their quality control AFTER the product is shipped? And does the 2nd or 3rd party supplier do any quality control checks?